How Can We Turn Ocean Water Into Renewable Energy
Saltwater could be used to produce greenish hydrogen using a system that combines electrochemical water splitting with forward osmosis. The arroyo could permit upwardly-scaling of hydrogen fuel production using the planet's predominantly salty natural h2o sources without pre-treatment or purification.
It's definitely one of those papers that makes you wonder, "How come no ane thought of that before?"
Mark Symes, Academy of Glasgow
Using solar free energy to electrochemically split up water into oxygen and hydrogen, akin to how plants photosynthesise, shows much promise for renewable free energy. The hydrogen that'south liberated can and so exist mixed with carbon dioxide to brand hydrogen fuels.
Nonetheless, efficient h2o splitting depends on catalytic electrodes, which usually require pure water at bones conditions to avoid damage. Scaling upward h2o splitting to ultimately produce hydrogen fuels has, therefore, been limited by the need for costly desalination and purification processes to obtain plenty pure deionised water. Meanwhile, most natural water sources are impure. Around 96.five% of the planet's water is stagnant or seawater, containing dissolved salts and organic affair that are corrosive to standard catalysts.
At present, Samuel Veroneau and Daniel Nocera at Harvard Academy, US, have shown how passive frontwards osmosis coupled with h2o splitting means catalysts tin can exist used with impure h2o sources with minimal losses in efficiency. 'The only surprising upshot for usa is that this had not been considered in detail previously, because all the work going on in water splitting,' says Nocera. 'Nearly piece of work focuses on pure h2o sources, not impure h2o sources.'
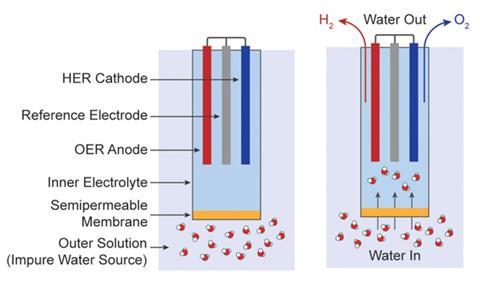
Nocera says he and his graduate student Veroneau had initially been looking at designing a new way to generate oxygen from seawater. In the process they noticed frontward osmosis could be effective in h2o splitting for energy uses, which led them to develop a frontward osmosis-water splitting (Fows) jail cell.
To make the experimental cell, the researchers cut off the bottom of a plastic centrifuge tube and turned it upside down. They then drilled a hole in the screw cap of the tube, at present at the bottom, earlier placing a frontwards osmosis semi-permeable cellulose acetate membrane inside the screw cap and screwing information technology on. Next they filled the upturned tube with a sodium phosphate electrolyte solution earlier inserting platinum electrodes to catalyse h2o splitting.
The team then prepared a sodium chloride solution to mimic seawater. When the Fows jail cell was placed inside the saltwater solution and a current practical, hydrogen and oxygen were produced. Equally the water in the cell depleted, a concentration gradient formed, enabling forward osmosis to bulldoze saltwater up through the predrilled hole in the tube's cap at the bottom and across the semi-permeable membrane to deliver pure water into the cell. Past balancing the influx and outflux rates, pure water could be continually extracted from the saltwater to feed the h2o-splitting cell.
'The beauty of the work is that there are no moving parts or extra energy inputs required here, merely an inexpensive semipermeable membrane,' comments Mark Symes, who investigates electrocatalysis at the University of Glasgow. 'Information technology's definitely i of those papers that makes y'all wonder, "How come no one thought of that before?" I would not be surprised to see rapid development of this thought into a big-calibration system for electrolysis in untreated water.'
How Can We Turn Ocean Water Into Renewable Energy,
Source: https://www.chemistryworld.com/news/seawater-splitting-system-could-scale-up-renewable-hydrogen-production/4013332.article
Posted by: lopezbricip1961.blogspot.com


0 Response to "How Can We Turn Ocean Water Into Renewable Energy"
Post a Comment